TGO 92 impacts on product name presentation
The new labelling legislation states that, as per TGO 92, ‘The name of the medicine on the main label must be presented in a continuous, uninterrupted manner and not be broken up by additional information or background text’.
Where the name of the medicine means the following:
(a) where the medicine is intended to be, or is, entered in the Register – the name of the medicine intended to appear or appearing on the Certificate of Registration or Certificate of Listing in relation to the medicine, not including the following information:
(i) the name of the active ingredient (except where the name of the active ingredient is intended by the sponsor to be, or because of the way it is represented, to form part of the name of the medicine);
(ii) the strength (except where numbers or words denoting strength are included in that name to differentiate medicines, by strength);
(iii) the dosage form (except where this is integral to differentiate medicines from other medicines);
(iv) container details;
(v) the pack size;
(vi) ‘new formulation’ or representations to the same effect;
(vii) flavour descriptors (except where this is integral to differentiate medicines from other medicines);
(viii) the name of the sponsor or distributor (or part thereof) (except where the name is intended by the sponsor to be, or because of the way it is represented, to form part of the name of the medicine);
This is likely to affect many sponsors if the Certificate of Listing lists their brand name as part of the name of the medicine, and for many will lead to the necessity to rebrand their entire range to accommodate the legislative requirements.
TGO 92 guidance documents give further information on what may or may not be permitted regarding the representation of the active ingredients and name on the main label.
The following are some examples of what has been stated as not permitted:
| Not permitted |
|
|
|
|
|
|
|
|
Applying the legislation
Below are examples of what might be considered non-compliant expressions of the name of the medicine according to TGO92. Please note that none of these products are sold in Australia they have simply been used to provide hypothetical examples.
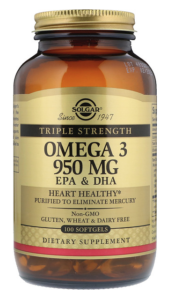
1.Eg. As per TGO 92, the name of the medicine on the main label must be presented in a continuous, uninterrupted manner and not be broken up by additional information or background text. As such if the name on the Certificate of Listing was ‘Solgar Triple Strength Omega 3 950mg’ then the following could be seen to be problematic:
- Solgar logo could be considered in breach of the labelling requirements for the name of the medicine, as it is not permitted to have distinguishing words, that are part of the medicine name, appearing in largely differing font where this results in the complete medicine name not being clearly discernible or easy to identify as a cohesive unit.
- Further logos or images between words that comprise the medicine name are not permitted, therefore the addition of ‘Since 1947’ and the concentric circles in the Solgar logo could be considered in breach.
- Additionally the words ‘Triple Strength’ separated from the rest of the name in a black banner and smaller font could also be considered in breach as the name of the medicine may be considered discontinuous due to words differing in both font style and size so that they are not linked.
- The entire name ‘Solgar Triple Strength Omega 3 950mg’ may not be considered a cohesive unit
2. Eg. As per TGO 92, the name of the medicine on the main label must be presented in a continuous, uninterrupted manner and not be broken up by additional information or background text. As such if the name on the Certificate of Listing was ‘Now Licorice Root 450mg’ then the following could be seen to be problematic:
- The ‘Now’ logo could be considered in breach of the labelling requirements for the name of the medicine, as it is not permitted to have distinguishing words, that are part of the medicine name, appearing in largely differing font where this results in the complete medicine name not being clearly discernible or easy to identify as a cohesive unit.
- Further logos or images between words that comprise the medicine name are not permitted, therefore the arch of translucent overlay and the use of the sun image within the ‘O’ and the use of the green sash across the label may be considered in breech.
- Also, the ‘450mg’ is in a different font size to the bulk of the name and may be considered disjointed.
- Additionally the spacing of the name from “now” to “Licorice” may be considered in breech as it separated onto more than one line with large spaces between the words, so that the words do not appear to be linked.
- The entire name ‘Now Licorice Root 450mg’ may not be considered a cohesive unit.
3.Eg. As per TGO 92, the name of the medicine on the main label must be presented in a continuous, uninterrupted manner and not be broken up by additional information or background text. As such if the name on the Certificate of Listing was ‘Bioray Kids NDF Happy’ then the following could be seen to be problematic:
- The ‘Bioray’ logo and the word “KIDS” could be considered in breach of the labelling requirements for the name of the medicine, as it is not permitted to have distinguishing words, that are part of the medicine name, appearing in largely differing font where this results in the complete medicine name not being clearly discernible or easy to identify as a cohesive unit.
- Further logos or images between words that comprise the medicine name are not permitted, therefore sunrays below the word Bioray could be considered in breech.
- The representation of the word Kids is presented in different lines that do not follow usual reading order.
- Also the interruption of the name with the additional text ‘Removes unwanted organisms & toxins’, disrupts the name and could be considered in breech.
- Furthermore TGO 92 states that ‘All text required by this Order to be on the main label must be oriented in the same direction.’ Both ‘Kids’ and ‘NDF’ would be considered in breech.
- The entire name ‘Bioray Kids NDF Happy’ may not be considered a cohesive unit.
The TGA has suggested:
‘If sponsors wish to maintain their house brand banners that may include images, they can of course repeat the house brand name in standard text with the remainder of the medicine name, or modify their logo such that the pictures are not between words e.g. move to the beginning of the cohesive unit or to the side‘ Please see below a mock up of how this might look.
Some of our sponsors have asked if they are able to remove their brand names from the Certificates of Listing to circumvent the above recommendation where brand names are going to be repeated consecutively on the main label. In short, the answer is in most cases no. This is due to TGA legislation stating that a product name must be unique, and therefore a brand name is likely to be required to provide a fully descriptive name that enables clear identification of the goods as they are presented for supply.