FREQUENTLY ASKED QUESTIONS
Looking for answers in other industries?
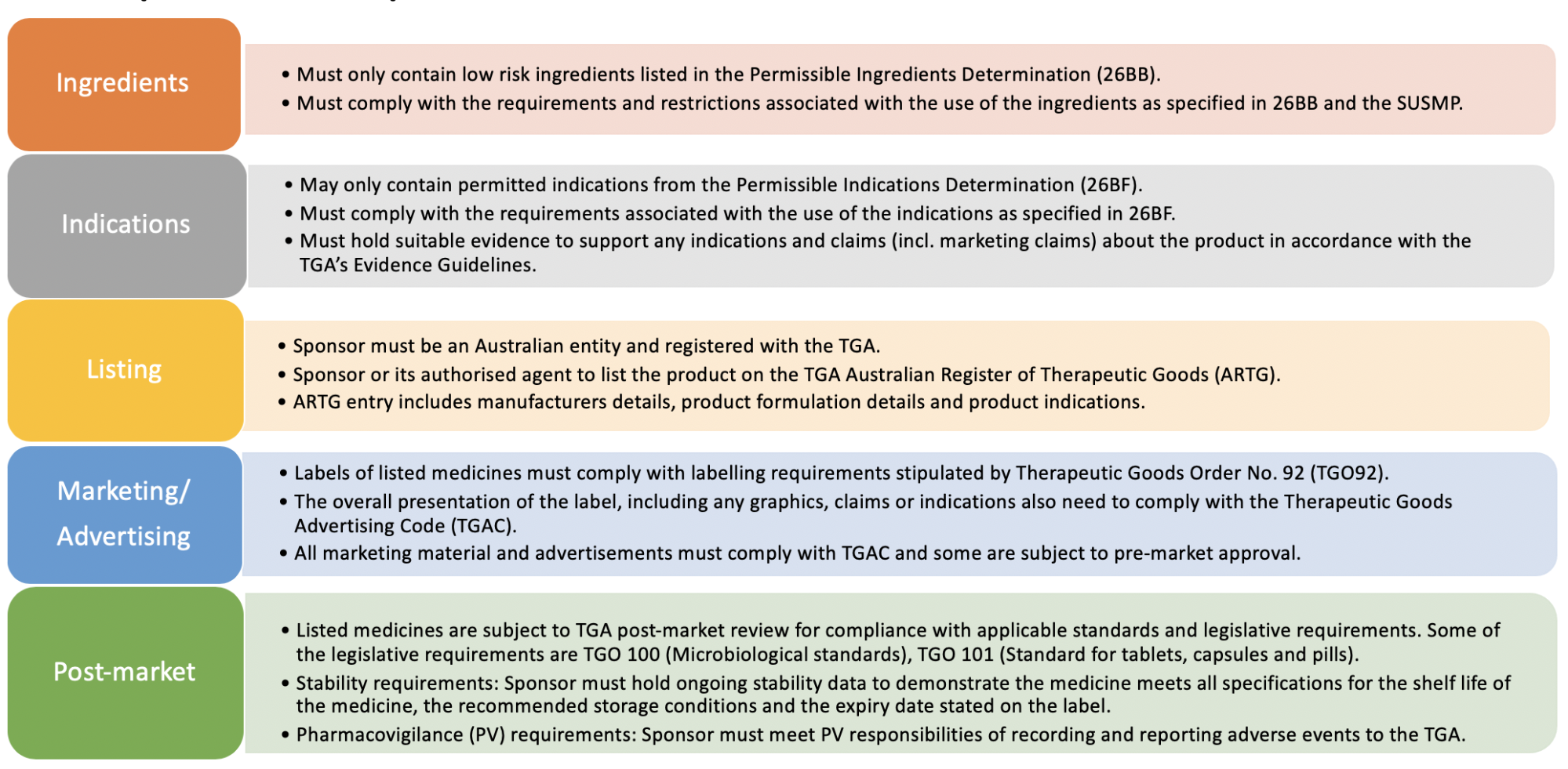
Listed Medicines are considered low risk medicines and are not required to show pre-market safety data and clinical trials. They are however required to be supported by suitable evidence for the claims that are being made and you are required to hold this evidence prior to listing the product on the Australian register of Therapeutic Goods (ARTG).
Our company can assist with all steps in the listing process. As a guideline for listed medicines the following steps are all the necessary steps in listing of products:
- Review of ingredients for compliance with regulations for listed medicines
- Review or preparation of product specifications
- Preparation of a full list of indications that can be used on your label artwork and in advertising and the evidence packages as per the current evidence guidelines
- Listing company as a sponsors with the TGA
- Preparation and submission of the application for listing
- Preparation of the label draft advising of all of the information needed to appear on the label
- Review of the label artwork prior to print
- Stability protocols and management
- Pharmacovigilance systems and training
We can also assist with:
- Market comparisons
- Product development
- Reformulation to comply with Australian requirements
In most cases ‘dietary supplements’ fall under the category of therapeutic goods in Australia, specifically listed medicines.
The first step, if you are wishing to import dietary supplements into Australia for sale is to confirm whether your products are manufactured in a TGA licenced facility or whether the manufacturing facility has Pharmaceutical GMP certification in a country with whom Australia has a mutual recognition agreement. To do this you need to speak with your manufacture to see if they have a TGA licence or Pharmaceutical GMP.
Please note that GMP for foods is not sufficient for listed medicines it must be pharmaceutical GMP.
Australia does not have a mutual recognition agreement with the USA.
If the products are to be imported and are made by a TGA licenced manufacturer or in a facility with Pharmaceutical GMP in a country with whom the TGA have a mutual recognition agreement you will require GMP Clearance from the TGA.
You will also need the ingredients in your products reviewed for compliance for use in listed medicines.
We can help you to apply for GMP Clearance and undertake ingredient reviews.
Please contact us for further information.
Below is a list of the basics
If you have already checked the TGA’s food medicine interface tool and are still unsure AWRS can assist you in understanding which category your product falls under.
We can certainly assist with your sunscreen.
In Australia, sunscreens fall into two categories based on their function as delineated by the Sunscreen Standard (and also based on how they are regulated): “primary sunscreens” (also known as “therapeutic sunscreens”) and “secondary sunscreens” (which cover both cosmetic sunscreens and some therapeutic sunscreens)
- primary sunscreens (those used primarily for protection of all parts of the body from UV radiation) are regulated as low-risk medicines by the TGA and must be listed in the ARTG.
- secondary sunscreens (products that contain sunscreening agents but whose primary purpose is something other than sunscreening) may, depending on their nature and SPF rating, be classified and regulated as medicines (in the same way as primary sunscreens) or be classified as cosmetics and regulated by NICNAS and the Australian Competition and Consumer Commission (ACCC).
Secondary sunscreens regulated as cosmetics by NICNAS and the ACCC include:
- moisturisers with sunscreen with SPF up to 15
- sunbathing products (eg oils, creams or gels, including products for tanning without sun and after sun care products) with SPF between 4 and 15
- make-up products with sunscreens, and
- lip-sticks and lip balms with sunscreens.
We have fixed prices for many of our services for listed medicines.
Anything not on our fixed price list is charged at an hourly rate in 0.1 hour (6 minute) increments.
We can certainly assist with food label reviews. To be able to provide a quote for food label reviews we need to see the labels.
This is a complicated space and it really depends on what you want to say about your product as to which category the product falls into.
There is no specific “functional foods” category in Australia. The product will most likely either be a Therapeutic Good (Listed Medicine) or alternatively a Formulated Supplementary Food or Formulated Supplementary Sports Food. Each category has different requirements from a formulation, permitted ingredient and claims perspective.
AWRS can assist with working out which category your product falls into based on your wish list of ingredients and the claims you are hoping to make and we can also assist with further formulation to ensure compliance with whatever category the product falls into and meeting requirements for claims.
Please contact us with your formulation details thus far and what you are hoping to say about it and from there we can advise on how we should proceed.
Teas can fall under either Food or Therapeutic Goods (Listed Medicines) regulations depending on the claims being made and sometimes the ingredients. If you are making therapeutic claims then they would be considered Therapeutic Goods and must comply with regulatory requirements for listed medicines.
If however they are simply herbal beautiful teas, that don’t rely on making therapeutic claims then they would fall under the Food regulations.
AWRS can review the ingredients in the products for compliance with the Food Standards Code as well as provide label drafts and label reviews.
Cosmetic regulations are governed by the National Industrial Chemical Notification and Assessment Scheme (NICNAS). Ingredients in cosmetic products, even those described as ‘natural’, are regulated as industrial chemicals under the Industrial Chemicals (Notification and Assessment) Act 1989 (the Act).
There is no certification for cosmetics however there are of course regulations/conditions around ingredients that can be used in cosmetics and also labelling laws around claims and warnings etc.
Regarding importation of cosmetics all importers of industrial chemicals for commercial purposes (including cosmetics) must register with NICNAS regardless of the amount of industrial chemicals imported in that registration year. The registration year runs from 1 September to 31 August in the following year. You can do it all through the NICNAS Business Services.
For cosmetics as a basic guideline AWRS can assist you with all steps in the compliance review process:
- Review of ingredients to ascertain whether they are permitted for use in cosmetic products and to advise which may require annual reporting or fit exemption categories for annual reporting.
- Review of the ingredients to ensure they do not come under a schedule of the SUSMP and if so what the labelling requirements are.
- Review of labels for claims compliance
- Preparation of annual reports and exemption forms
- Registering with NICNAS



